The company
PanPath B.V. is a privately owned company founded by scientists with a background in pathology and molecular biology. This knowledge is combined with a broad experience in the environment of commercial companies active in antibodies, detection systems and molecular biology. PanPath B.V. is based in Budel, The Netherlands. The company was founded in 2002 and started with the introduction of the REMBRANDT® product group. The brief history of PanPath is marked with the launch of a broad range of well-designed products, a world-wide distributor network and a corporate image of quality and reliability. The innovative R&D has resulted in an improvement of the REMBRANDT® product group and the addition of new products for the detection of cancer related genetic markers.
PanPath products are designed for ease of use. The high production standards are combined with a continuous Quality Control for each product in order to ensure reproducible results with minimized lot to lot variation.
PanPath distributors are competent and knowledgeable, and we keep them regularly informed on all aspects of the technology and applications of our products.
Mission
PanPath B.V.’s mission is the development, production and sales of innovative diagnostics for the pathology market with support from a number of scientists located at different research institutes, worldwide. PanPath strongly believes in co-operation with scientists who are active in scientific research in order to be kept aware of the needs encountered in daily routine.
Vision
PanPath B.V. aims to become a leading manufacturer of diagnostic assays for the pathology market. We aim for better, more reliable and quick diagnosis for patients.

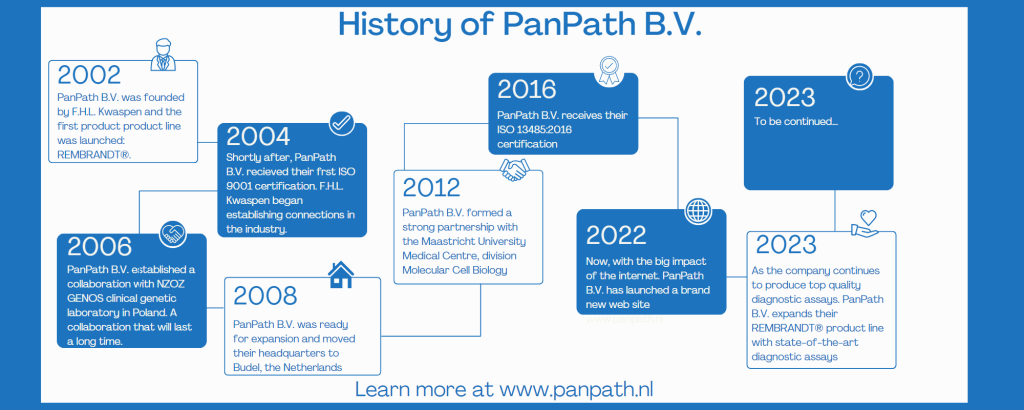
History
Co-operations
NZOZ GENOS s.c. Lodz, Poland
PanPath is working together with GENOS since 2006. GENOS is a medical laboratory that specializes in clinical genetics. GENOS also has an active role in execution of performance studies for PanPath B.V.
Maastricht University Medical Centre (MUMC), division molecular cell biology
Since 2012 the MOLCELB division of MUMC has a supporting partner in product development for PanPath B.V.
Maastricht University Medical Centre (MUMC), division pathology
To hold up to standards of the in vitro diagnostic regulations (IVDR), PanPath works together with the pathology division at MUMC for the execution of clinical performance studies.



